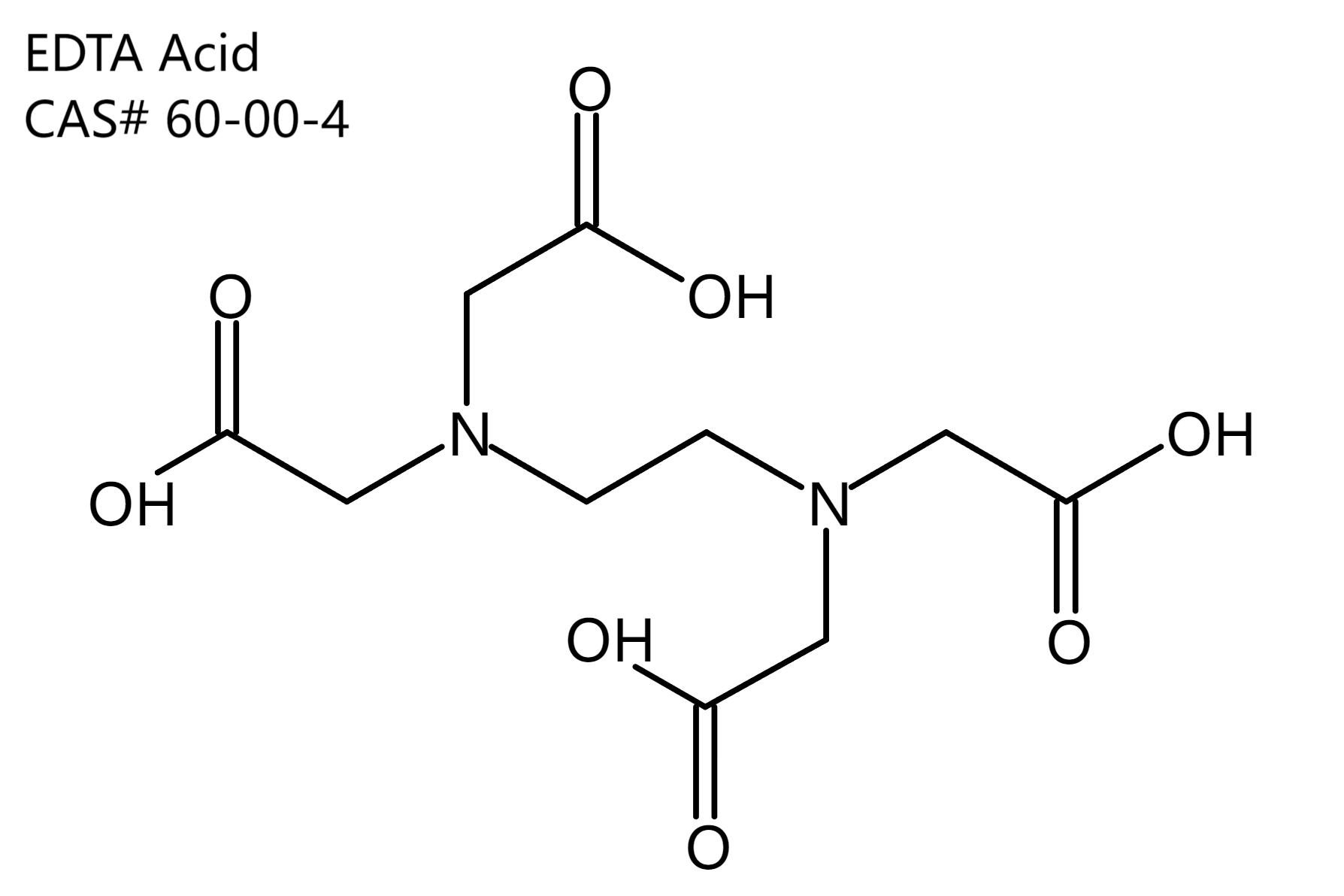
Ethylene diamine tetraacetic acid (EDTA)
Product name: EDTA
Molecular formula: C10H16N2O8
Molecular weight: M=292.2
CAS number: 60-00-4;
EC number: 200-449-4
Synonyms:
Edetic acid;
Glycine, N,N’-1,2-ethanediylbis[N-(carboxymethyl)-;
Ethylenediaminetetraacetic acid;
EDTA is a representative substance of chelating agents, and various metal salts of EDTA are often used in practical applications.
Similar Products
EDTA sodium salts: EDTA-2Na, EDTA-4Na, EDTA-3Na
EDTA potassium salts: EDTA-2K, EDTA-3K
EDTA chelated fertilizer
Technical Data
| items | EDTA Acid |
|---|---|
| Appearance | White crystalline powder |
| Content [%] | ≥99.0 |
| Chloride (calculated as Cl) [%] | ≤ 0.05 |
| Sulfate (calculated as SO4) [%] | ≤ 0.05 |
| Heavy metals (calculated as Pb) [%] | ≤ 0.001 |
| Iron (calculated as Fe) [%] | ≤ 0.001 |
| Chelation value [mgCaCO3/g] | ≥ 339 |
| PH value | 2.5-3.0 |
| Characteristic | EDTA acid is soluble in alkaline solutions, such as solutions of sodium hydroxide, sodium carbonate, and ammonia. Insoluble in cold water, alcohol and general organic solvents. |
Uses
EDTA has a wide range of uses and is a representative substance of chelating agents, complexing agent, sequestering agent. EDTA acid can form stable water-soluble complexes with alkali metals, rare earth elements, and transition metals.
EDTA is widely used in polymer chemistry industry, daily chemical industry, paper industry, pharmaceutical industry, agriculture, textile printing and dyeing industry, aquaculture, photographic chemicals, oilfield chemicals, water treatment agents, boiler cleaning agents and analytical reagents.
EDTA Acid is insoluble in cold water and slightly soluble in boiling water, but soluble in alkaline solutions, such as sodium hydroxide.
EDTA sodium are easily soluble in water and are often used in various aqueous solutions.
Related Products
Traditonal Chelating Agents
Aminopolycarboxylates: EDTA acid, EDTA-Na/K, DTPA & DTPA-5Na/5K
Polyphosphonates: HEDP, DTPMP, ATMP, PBTCA
Properties
EDTA acid is a white crystalline powder with a melting point of 240℃ (decomposition); at 150℃, it shows a tendency to remove carboxyl groups.
EDTA acid is soluble in alkaline solutions, such as solutions of sodium hydroxide, sodium carbonate, and ammonia.
EDTA acid is insoluble in cold water, alcohol, and general organic solvents. The solubility in water is only 0.5g/L (25℃).
Slightly soluble in hot water, soluble in 160 parts of boiling water.
Packaging
25kg/bag or according to customer requirements
Store in a dry and ventilated warehouse, avoid direct sunlight, and stack gently.